Do NAD precursors work? It may be the wrong question. Interview with Nuchido founder Dr Nichola Conlon
Does Nicotinamide Mononucleotide work in humans?
Readers of the blog are well aware, and in rapt attention, as our Founder Nick personally delves into the self-experimentation required to answer this question - Does NMN work? In just under two weeks, he will re-test his biological age after a 6-month NMN led protocol, using the most advanced and detailed epigenetic aging clock available.
The aim - get to the bottom of what many thousands of you are asking - should I be taking a NAD precursor?
As A Longer Life has continued to review the latest research on NAD boosting strategies for enhanced longevity, a number of important discoveries have emerged, which can broadly be grouped into two categories.
First, the absorption and effectiveness of Nicotinamide Mononucleotide in humans is still hotly contested.
If you cite the research from advocates of Nicotinamide Riboside (NR), you’ll note objections about the lack of clinical research for NMN and how NMN ultimately must first be converted into NR prior to entering into the cell.
Now most of these folks are financially involved in NR’s success (which is a patented molecule), so we must consider the potential role this will play in their personal biases.
Ultimately, we owe it to NMN to allow it to pass through the rigour of clinical trials, of which a growing number are now underway.
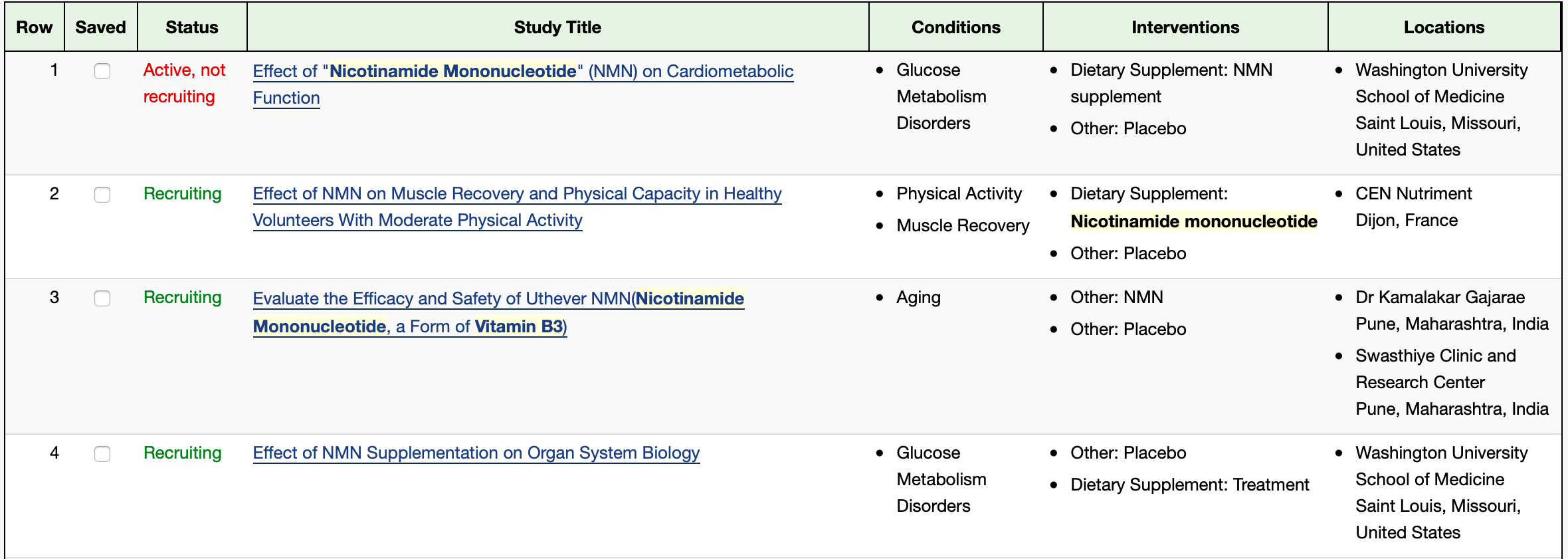
The list of NMN clinical trials continues to expand. Review them yourself on clinicaltrials.gov
Secondly, it is clear that the NAD precursor approach to NAD boosting in the cell is actually a bit myopic.
The NAD system, like all things in biology, is much more complex than our favourite supplement company’s marketing bullet points.
The layperson could be forgiven for overlooking this complexity, but we think it is incredibly important to explore it in an approachable form.
Enter NAD systems expert Dr Nichola Conlon
As A Longer Life explored the complexities of NAD systems biology in greater detail, the expertise, charming accent and straight-forward explanations of Nuchido founder Dr Nichola Conlon emerged as a bright spot in the perhaps oversimplified NAD precursor debate (NMN vs. NR vs. Nicotinamide, etc).
One particularly valuable point of reference was Dr Conlon’s explanation of the ‘multi-targeted approach’ her company has developed (led by her own expert background) to the NAD boosting challenge, presented at Undoing Aging 2019:
To dive deeper into this complexity, including how a more nuanced understanding of NAD biology can help us enhance our approach to boosting it to more youthful levels, A Longer Life conducted a zoom interview with Nichola in February 2021. Let’s dive into Part 1…
Interview with Dr Nichola Conlon of Nuchido: Part 1 - NAD systems biology
A Longer Life (ALL):
Readers of the A Longer Life are familiar with NAD precursors, however, what may be new information is how taking precursors can be equated with a single targeted approach. You’ve previously spoken about this on many occasions, could you introduce the reader to this concept? What do you mean by a single targeted approach?
Dr Nichola Conlon (NC):
So first of all, biology is extremely complex and complicated. And nothing in biology ever exists alone, or in isolation. Everything that is going on within our bodies and in our cells, is actually very interconnected and intertwined. For example, there are multiple pathways that are running alongside each other performing the same job, and there are multiple pathways that are talking to each other. There are pathways, proteins and genes that are feeding information to each other all the time, and may be also activating or inhibiting each other and other processes.
Another very important concept for your readers to appreciate, is that, in order for scientists to understand and analyse biology, it is normal for them to pick these complex processes, for example, they might do experiments on a particular protein or a particular pathway, and then write a scientific paper on it. Overall, this is fine, because it helps us to understand that small piece of biology. But problems arise when you try to interpret those results in isolation without the wider context - you've got to appreciate that a single protein or pathway in the body is a very small part of a much bigger picture.
ALL: What you are describing is precisely what science has done with NAD to date. What is in our mind is the classic chart of our NAD levels declining with with age - the natural conclusion becomes “We need to boost NAD levels”, and that's where much of the early research has focused. But what you’re very clearly pointing out is - it's not that simple.
NC: Yeah, that's it. All of the science says, ‘OK, NAD declines over age’, so the most obvious response is ‘how do we boost NAD?’. So at first sight, it would seem that taking a NAD precursor is a good idea.
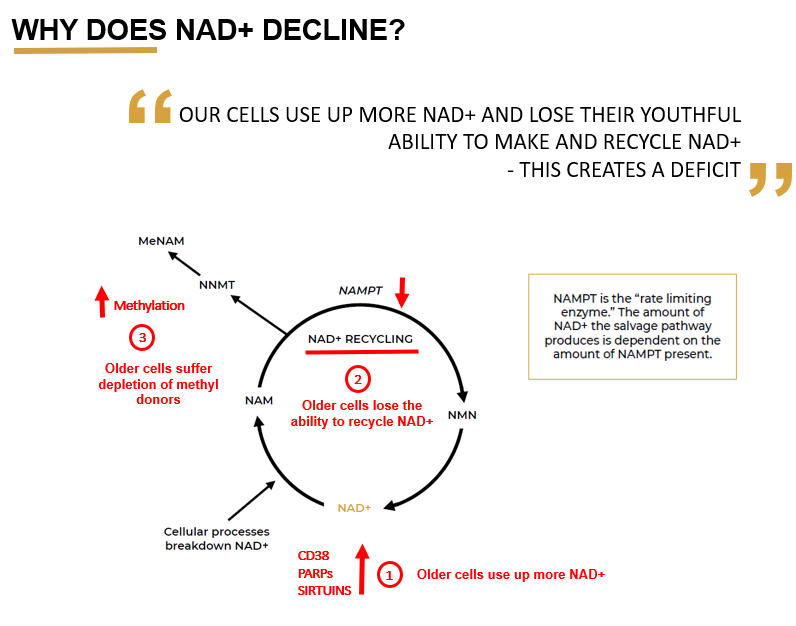
These NAD precursors are basically the raw material that the cell needs to make NAD. But the problem is, it's now known that NAD doesn't decline because your cells have a shortage of the raw material to make NAD. Rather, it's actually declining because there are multiple other things going on in the cell causing its decline. For example, the cell is losing its ability to manufacture and recycle NAD.
 |
Image credit: Nuchido
ALL: You have kindly provided a really nice graphic (pictured) showing why NAD declines with age, within which this ability to make and recycle NAD is declining over time. Let’s focus on the major components in this graphic. Can we walk through each of those points and have you provide the reader with the basic concepts?
NC: The first point is that old cells use up more NAD. The reason for this is that they have more damage and inflammation, and so they need more repair. NAD is also a substrate for important repair pathways in the cell that are trying to fix this damage. What this means is that the enzymes and repair processes are turned right up, and whilst performing their function they’re consuming huge amounts of NAD.
ALL: This consumption of NAD by repair processes is discussed broadly in the anti aging community, including compounds such as resveratrol, and its role in activating sirtuins. Could you elaborate on how aging impacts how these repair processes consume NAD?
NC: DNA repair enzymes use NAD as a substrate to drive their activity, and these activities start to increase as our cells get older - which means aging cells are actually consuming more NAD. In a young cell, this NAD consumption is not actually a problem, as young cells have a very good ability to make their own NAD via recycling. So when NAD is used up in young cells it is broken down into nicotinamide and they have the ability to recycle this straight back into fresh NAD again. This means the cells are simply recycling the same NAD time and time again.
ALL: This recycling process, also commonly known as the ‘salvage pathway’, is #2 in the provided image. There are a few key inputs to this pathway, one of those is Nampt - walk us through it.
NC: Correct, this is the salvage pathway. And that's why it's called the salvage pathway - it is salvaging NAD. This pathway relies on an enzyme called Nampt, which is known as a ‘rate limiting enzyme’. This means that it is the bottleneck in the process. When the levels of this enzyme go down, it slows down the recycling of nicotinamide back into NAD.
What's been discovered recently, is that one of the main reasons that NAD is declining in cells as we get older, is because the Nampt enzyme declines with age. This means that older cells are less able to recycle NAD. And this is problematic because, as I have already mentioned, in older cells NAD is getting used up more quickly (#1, as discussed previously), because of increased activity of the damage repair systems. So large amounts of NAD is being used up and broken down in nicotinamide, which has the capacity to be recycled - but its not.
This is because with Nampt enzyme levels declining, the recycling process can’t keep up. So the nicotinamide starts to build up and rather than being recycled back into fresh NAD. So right at a time when our cells need this recycling process to work really well, it's actually declined. This creates a deficit in NAD, and from there, quite an exponential drop in NAD levels.
ALL: That’s a great segue into part #3, which relates to methylation. Methylation plays a collaborative role with the recycling pathway of NAD.
NC: The body wants to keep a very tight handle on the levels of anything the cell considers a waste product. In the cell, when NAD is used up and broken down, for example by PARPs, sirtuins, etc the output is nicotinamide. If the recycling pathway is working well, it can be recycled straight back into NAD. But when it doesn’t work well (i.e. in aging), nicotinamide can build up and the cell essentially says “Oh my goodness, and there’s a buildup of nicotinamide in the cell, we need to get rid of it!”
The cell does this by increasing expression of another enzyme called NNMT, which adds a methyl group to nicotinamide, creating methyl nicotinamide. This acts as a signal to tell the cell to excrete it, which helps the cell to get rid of this excess nicotinamide from the NAD breakdown.
So what you find is that in older cells, there is increased expression of the NNMT enzyme because the cell is actively trying to remove excess nicotinamide, because it is no longer being recycled. The consequence of this is that your cells end up using a lot of methyl groups to process it. Methyl groups are also very important in other areas of biology such as epigenetics, so this has a knock on effect and the result is methyl donor depletion, meaning that the cell doesn’t have available methyl groups for those other important processes.
This is a great example of how everything's really interlinked in biology, and you can't look at any of these processes in isolation.
ALL: This complexity is one of the reasons why A Longer Life was so interested to speak with you about the NAD system in more depth. One of the things that you had spoken about previously, was this idea of there being anabolic and catabolic elements of the NAD cycle. As a reminder for the reader, anabolic means building up, and catabolic means breaking down. How do these terms relate to this image that we're looking at now?
NC: The main anabolic enzyme is Nampt, because that is the main way that cells replenish their NAD - by recycling the breakdown product, which is nicotinamide. The main driver on the catabolic side, or breakdown of NAD, which we haven't spoken about yet is CD38. CD38 is another really important part of the puzzle.
ALL: Discussion in the longevity community on CD38 is certainly picking up, with more compounds for inhibiting CD38 becoming available. Talk to us about CD38, our readers are quite curious to dive into this topic as well.
CD38 via Wikipedia
NC: CD38 is a membrane protein, which basically acts as an enzyme that uses NAD as a substrate to produce cADPR, which is a secondary messenger for the cell. CD38 expression is found to massively increase with age and the main problem with this is that CD38 uses a lot of NAD. To create just one molecule of its downstream messenger, it has to metabolize around 100 molecules of NAD. This means that even relatively small increases in the levels of CD38, result in a massive decrease in the amount of NAD available to the cell. Inhibiting CD38, can increase cellular NAD levels by 50%, which shows what a huge impact CD38 actually has.
ALL: Now we're starting to really understand the difference between the ‘single targeted approach’ of adding more NAD precursor (e.g. NR, NMN) into the cellular system, and a ‘multi targeted approach’ which considers CD38 levels, as well as NNMT, Nampt, etc.
Clearly, we need to think about this in any NAD boosting strategy, as well as managing methylation. How do we engage with each of these components to raise cellular NAD to more youthful levels, as we grow older and the systems we discussed are starting to break down?
NC: Exactly, a multi targeted approach is what Nuchido is all about, because nothing in biology exists in isolation. I think just one thing just to point out, which will probably be interesting for your readers is just looking at it from the point of view of what happens if you are only taking a precursor?
ALL: Let’s absolutely discuss this, Nichola, at the moment, A Longer Life is running a self-experiment with the NAD precursor NMN. This includes a before and after biological age test with UK based Chronomics, and will conclude at the end of February 2021.
A Longer Life’s self-experiment with NMN is powered by DoNotAge. Use the code ‘longevityblog’ to save 10% on any order.
The supplement stack I am testing is the popular NMN + Resveratrol + Tri-Methyl Glycine combo from David Sinclair's Lifespan book, which many folks around the world are now taking. This is why A Longer Life is running this experiment - to see if it can impact biological age, as Dr. Sinclair seems to suggest.
NC: We'll use that exact protocol as an example. You are adding NMN into the system, which will be converted in the cell to NAD. This NAD will then be used up in the cell by processes that rely on NAD such as your DNA repair enzymes and also the sirtuins, which you're also activating with the resveratrol that you mentioned. It will also be consumed by CD38 (#1), and all of these processes cause the NAD to be broken down into nicotinamide.
This is where the problem begins, especially in older people, because the levels of the Nampt enzyme which would usually recycle this nicotinamide back into NAD (#2) have declined. So by taking an NAD precursor, you've put NAD into the system, but it only has the opportunity to be used once, when really the cell could keep recycling that precursor back into new NAD again, if it had an efficient salvage pathway, but in older cells, it doesn't.
NAD precursor supplementation can deplete the Methyl donor pool - learn more about this risk in our previous post on NMN safety and risk management.
The outcome of this failure to recycle results in a buildup of nicotinamide in the cell. Now we’re back to the methylation problem (#3) because the cell has all this new nicotinamide hanging around. It can't convert it back into NAD because Nampt isn't working as well as it should be so it needs to get rid of it, so it increases expression of the NNMT enzyme which methylates nicotinamide to help excrete it from the cell. And the resulting problem is a reduction in methyl groups in the cell. Which is exactly why you are taking tri-methyl glycine (TMG) alongside NMN. Because it's known that people taking precursors have a big problem with methyl donor depletion, and TMG contains three methyl groups and a glycine to try to replenish this.
The key point is that you're never addressing the root of the problem and instead by effectively trying to paper over the cracks you are creating other issues. And actually, if you just fixed the recycling problem, then you wouldn't have that issue with methylation because the levels of nicotinamide would never build up so big that they need to be methylated and excreted, it would just get recycled.
ALL: Brilliant explanation Nichola. So would you say that with the precursor supplement approach that we're on the right track, but we're missing some components? Or is the precursor led approach fundamentally flawed?
NC: Precursors were the first solution that was available based on the best available information at the time. But the scientific understanding has developed since then. We now know the underlying reasons why NAD declines with age, and we know how we can fix them.
People who are experimenting should always keep re-evaluating what they're doing - asking themselves ‘is this still a sensible approach based on what the science is saying?’
As we age, the NAD factory is losing function
I often ask people who are taking NAD precursors to think of it in this way - imagine that your cells are little NAD factories, and and all of a sudden, the production of NAD in this factory declines. You find that the reason that NAD production has gone down is because the machines have become old and broken and the factory pipes are leaking, wasting material. In this scenario, do you think it would be a good idea to try and boost production in this factory, simply by ordering more raw material to be delivered to the factory gates?
Of course not! If you want to boost production again you are going to have to fix the factory first, otherwise the raw material will just pile up. But this is exactly what the precursor approach to boosting NAD does - it ignores that the cell’s NAD enzymes aren’t working like they used to and that proteins such as CD38 are wasting NAD and just piles more raw material into the cell. But if you want to have any real impact you need to address the root causes.
ALL: It's a wonderful illustration. And I'm glad you shared that with us. In doing my background research for this interview, I’d heard you use it before, and I thought it was a very appropriate way to visualise the problem.
Nichola, let's start thinking about how to repair that factory. This is where I see an innovative approach out of Nuchido, which extends from some of your own research background. Tell us what approach you’re taking at Nuchido to restore the NAD ‘factory’ back to youthful levels…
FDA & TGA DISCLAIMER
This information is intended for educational purposes only and is not meant to substitute for medical care or to prescribe treatment for any specific health condition. These blog posts are not intended to diagnose, treat, cure or prevent any disease, and only may become actionable through consultation with a medical professional.